 Here’s an update on the recent financial upheaval in the world of dialysis reimbursement. The expanded bundling payment system was officially implemented in January 2011, and most dialysis units have now switched over to this new billing practice.
Here’s an update on the recent financial upheaval in the world of dialysis reimbursement. The expanded bundling payment system was officially implemented in January 2011, and most dialysis units have now switched over to this new billing practice.
Wednesday, August 31, 2011
Bundling Redux
 Here’s an update on the recent financial upheaval in the world of dialysis reimbursement. The expanded bundling payment system was officially implemented in January 2011, and most dialysis units have now switched over to this new billing practice.
Here’s an update on the recent financial upheaval in the world of dialysis reimbursement. The expanded bundling payment system was officially implemented in January 2011, and most dialysis units have now switched over to this new billing practice.
Thursday, August 25, 2011
MPGN: Think Different
My main gripe with the existing classification system is that it places the focus firmly on “primary” causes of MPGN (Types I, II, III, Burkholder variant, Strife and Anders variant etc.), demoting secondary causes to the bottom of the differential. Although idiopathic forms undoubtedly exist, they are increasingly uncommon, and an underlying cause can be found in the vast majority of cases.
First off, it is vital to appreciate that MPGN is not a disease, but a pattern of injury with a broad differential. For a nephrologist to say their patient “has MPGN” just doesn’t cut it, no more than an ID doc saying their patient “has infection”. The classic presentation is a child or young adult with persistent proteinuria, often with overlapping features of the nephrotic and nephritic syndromes, and depressed C3 (occurs in over 80%). Spontaneous remission is rare and the condition typically grumbles on to ESRD over many years, unless a treatable underlying cause can be found. The hallmark biopsy finding is the double contour on light microscopy (adjacent figure). The double contour may be thought of as the fingerprint of chronic and persistent immunoglobulin/complement-mediated endothelial injury. Endothelial and mesangial cells attempt to repair the ongoing injury by generating basement membrane–like material, which traps immune complexes and cellular elements in between to form double contours.
The proposed classification system is similar to that used for rapidly progressive or crescentic GN, where classification by immunoflourescence pattern on the the renal biopsy into linear, granular and pauci immune staining is extremely helpful in narrowing down the equally broad and complex differential. The proposed MPGN classification divides it into 3 IF pattern groups: Immunoglobulin staining with C3, C3 staining without immunoglobulin and no staining (figure below; modified version of that which appears in article).
Immunoglobulin and C3 +ve: If immunoglobulin staining is present, the patient should be worked-up for infection, monoclonal gammopathy and autoimmune disease. Regarding infective causes, the organisms to think about tend to be classically associated with chronic infection (HCV, HBV, TB, coxiella, brucella, nocardia) or a carrier state (mycoplasma, Neisseria, strep), presumably acting as a source of chronic, low-grade antibody production. When infective and autoimmune causes are excluded, an underlying MGUS is found in over 40% of cases (Mayo clinic series). This is best identified by immunofixation, although IF microscopy on the biopsy specimen using monoclonal protein–specific Abs is often very helpful. Less common associations include myeloma, lymphoma and CLL.
C3 +ve and immunoglobulin -ve: Isolated C3 staining (i.e.without immunoglobulin) suggests an abnormality of the alternative complement pathway. When this pattern occurs in children, it suggests a genetic mutation is responsible, whereas complement autoantibodies are more likely to be responsible in adults. The prototypic diseases are dense deposit disease and MPGN with isolated C3 deposits, however there are many others. A detailed interrogation of the alternative complement pathway will probably require an immunologist’s input. However, before calling them, a good initial screen of the alternative complement pathway comprises 4 tests: C3 and C4 levels, serum MAC levels, an alternative pathway functional assay (APFA) and hemolytic complement assays.
No staining: Chronic injury to the endothelial cell due to thrombotic microangiopathy also causes an MPGN pattern. However, staining for immunoglobulin and C3 is absent. Causes to consider include TTP, HUS, anti-phospholipid antibody syndrome, drug-associated TMA, nephropathy associated with bone marrow transplantation, radiation nephritis and malignant hypertension.
Hopefully, using this system, you will be able to quickly hone in on a manageable differential diagnosis, leaving you more time to... think.
Surviving Stress in the Kidney Medulla
 Hypertonicity in kidney medullary interstitium is essential for urinary concentration. Osmolality in the papillary interstitium can reach up to 1200 mOsm/kg. How do cells in the renal medullary interstitium survive this hypertonic stress?
Hypertonicity in kidney medullary interstitium is essential for urinary concentration. Osmolality in the papillary interstitium can reach up to 1200 mOsm/kg. How do cells in the renal medullary interstitium survive this hypertonic stress?HemoDoc in AJKD
 Also in this months issue of AJKD is a very well written piece by Dr Peter Laird of HemoDoc and Dialysis from the sharp end of the needle in the In a Few Words section on his own experience with chronic kidney disease and home hemodialysis. It's fascinating to see web authors starting to appear in traditional media.
Also in this months issue of AJKD is a very well written piece by Dr Peter Laird of HemoDoc and Dialysis from the sharp end of the needle in the In a Few Words section on his own experience with chronic kidney disease and home hemodialysis. It's fascinating to see web authors starting to appear in traditional media.
Wednesday, August 24, 2011
RFN bloggers in AJKD
 Shameless plug. Check out the just released AJKD article, Embracing the Internet as a Means of Enhancing Medical Education in Nephrology by Matt and Conall along with Kenar and Sidharth from Nephron Power.
Shameless plug. Check out the just released AJKD article, Embracing the Internet as a Means of Enhancing Medical Education in Nephrology by Matt and Conall along with Kenar and Sidharth from Nephron Power.
Tuesday, August 23, 2011
AKI requiring dialysis in the hospital: Does this mean I'm on dialysis for good?
 As nephrology fellows we see a lot of Acute Kidney Injury (AKI) on the inpatient consultative service. When AKI in the hospital requires dialysis people and their families naturally want to know whether this means long term dialysis will be required.
As nephrology fellows we see a lot of Acute Kidney Injury (AKI) on the inpatient consultative service. When AKI in the hospital requires dialysis people and their families naturally want to know whether this means long term dialysis will be required.
The answer, as with many things in medicine, is it depends. AKI has a wide variety of causes but one of the most commonly encountered entities in the hospital is Acute Tubular Necrosis (ATN) and luckily we have a number of good studies to help inform prognosis.
One of the first things to realize about hospitalized AKI due to ATN that requires dialysis is that it is associated with a very high in-hospital mortality rate. In the ATN study which examined critically ill ICU patients with AKI presumed secondary to ATN randomized to either intensive or less-intensive dialysis there was a roughly 50% in-hospital mortality in both groups.
In those with ATN who require dialysis and survive hospitalization, whether or not long term dialysis is required depends on the demographic. In general, the lower the baseline kidney function at the time of AKI the higher the rates of long term dialysis dependence.
The ATN study excluded patient's with advanced CKD (about 30% of patients though had moderate CKD with GFRs between 30 and 59 ml/min/1.73m2). Around 70% of people who survived to day 28 continued to require dialysis. In contrast, in a German cohort of 433 critically ill patients all with GFRs of greater than 90 ml/min/1.73m2 who developed dialysis requiring AKI from ATN not one survivor (in hospital mortality was again approximately 50%) required long term dialysis at discharge.
In terms of more advanced CKD, a study using the Northern California Kaiser Database looked at patients both in and out of the ICU who developed AKI requiring dialysis (greater than 90% of patient's had likely ATN by subset chart review). Of those that survived the hospitalization (overall mortality was 26%) 42%, 63% and 90% of patients with eGFRs of 30-44, 15-29 and less than 15 ml/min/1.73m2 respectively were felt to be dialysis dependent within 30 days of hospital discharge (a selected chart review revealed no cases of recovery within three months).
In summary those with AKI secondary to ATN who require dialysis in the ICU have a very high mortality rate. Of survivors, approximately 70% will require long term dialysis unless they enter the hospital with completely normal renal function in which case the chances of renal recovery appear to be quite good. Patient's with the most advanced form of CKD who develop AKI requiring dialysis are very unlikely to recover.
Monday, August 22, 2011
A spoonful of sugar...

Cholera epidemics have been a major public health issue throughout recorded history and much energy was devoted towards preventing them and treating affected patients once they occurred. However, it was not until 1831 that it was recognized that the deaths were due to a loss of both salt and water in the stool. At the same time, it was shown that salt and water alone orally or by enemas was ineffective and potentially harmful and iv fluids were introduced for the treatment of cholera for the first time. Despite this, mortality remained very high and as late as 1906, 40% of infected patients still died. Intravenous fluids remained the mainstay of treatment for diarrheal infections until the 1970s.
Although the Indian physician Sushruta had described using solutions containing rice water and carrot juice to treat diarrhea more than 3000 years ago, these had not been in vogue in the West. In the 1960s, small scale studies were done showing that oral solutions containing salt alone were not absorbed but that if the salt was combined with glucose, absorption of both was enhanced. This led to an increase in the use of oral rehydration therapy through the 1960s although it was not fully accepted as a replacement for iv fluids at that point. The first major use of ORT was in a camp for Bangladeshi refugees in 1971. The doctors ran out of iv fluids and ORT was used to treat more than 3000 patients with mortality falling from 30% to 3.6%.
So why is ORT so effective in the treatment of diarrhea in general and especially cholera? The cholera toxin binds irreversibly to the luminal membrane of the small intestine leading to activation of protein kinase A and ultimately increasing the number of open Cl channels on the luminal membrane. The exodus of Cl is accompanied by loss of Na through the tight junctions between the cells and so patients can become dehydrated very rapidly. ORT works because of the particulars of glucose absorption in the small intestine. The glucose transporter SGLT1 requires 2 Na in order to absorb one glucose molecule. ORT contains equimolar amounts of Na and glucose. Thus, the absorption of 100mmol of glucose requires the absorption of 200mmols of NaCl. The second 100mmols of NaCl comes from intestinal secretions and this amount is equivalent to about 700mls of isotonic secreted fluids. Thus, not only does ORT provide rehydration itself, but it also actually decreases diarrhea volume and reduces the requirement for iv fluids.
This was one of the major public health advances of the 20th century and has significantly reduced mortality from diarrheal illnesses worldwide. ORT solutions are cheap and widely available and, most importantly, are easy to administer with no training. Knowing your electrolyte transport mechanisms is important.
This is a great review of the past and future of ORT and this is a previous post by Nate comparing ORT and Gatorade.
Wednesday, August 17, 2011
Desensitization in kidney transplantation: a clear survival benefit
 I would like to direct your attention to a landmark paper in transplantation just published on the NEJM two weeks ago.
I would like to direct your attention to a landmark paper in transplantation just published on the NEJM two weeks ago.
We have previously discussed the terms used to characterize sensitized patients and the most common protocols to overcome this barrier to transplantation. Briefly, more than 30% of patients on the waiting list for a kidney transplant are sensitized to HLA antibodies, with more than 8,000 patients being highly sensitized. Sensitized patients have 3 options to undergo kidney tx:
- stay on the waiting list (hoping to get a matched kidney - annual transplantation is below 7%);
- enroll in a paired kidney donation (improves chances but still low rate); or
- undergo a desensitization protocol.
Desensitization usually involves plasmapheresis, IVIg and high dose immunosuppressive drugs (+/- rituximab) in order to decrease circulating anti-HLA antibodies. These patients have a high risk of complications, including bleeding, cancer, infection and antibody-mediated rejection (which is associated with poor allograft function). Moreover, these patients are very expensive to the hospital’s transplant program. More recently, with the spread of quality of measures in transplantation, having many sensitized patients on a program can significantly reduce the successful statistical outcomes of transplantation and consequently deteriorate the image of the program. Therefore, many programs just avoid taking highly sensitized patients.
Despite all the potential complications, whether undergoing desensitization leads to significant long-term survival benefit is unknown. Montgomery et al. analyzed a single-center cohort of 211 sensitized patients who underwent desensitization (IVIg+plasmapheresis) followed by renal transplantation, comparing with two carefully matched control groups of patients on a waiting list for kidney tx who continued to undergo dialysis (dialysis-only group) or who underwent either dialysis or HLA-compatible transplantation (dialysis-or-transplantation group). During the 11-year study period, 98% of the sensitized patients underwent successful transplantation and this study included patients with different detection methods of anti-HLA antibodies, such as positive cross-match by cytotoxicity assay, flow cytometry and/or bead assay (Luminex).
In the desensitized group, rates of survival were 90.6% at 1 year, 85.7% at 3 years and 80.6% at 8 years, compared with 91.1%, 67.2% and 30.5% in the dialysis-only group (view figure). Among the different levels of anti-HLA antibodies, patients with positive cross-match by cytotoxicity assay carried the worst outcome. Nonetheless, the survival benefit curve crossed at 18 months even in that group. Combining all sensitized groups, the survival benefit was clear after 12 months.
Overall, major adverse events during desensitization treatment occurred in less than 5% of patients, including anaphylaxis or bleeding. The most common cause of death was cardiovascular disease (~16% patients) and there were 6 deaths related to infection (~3%), which were likely secondary to the intensive immunosuppression produced by the desensitization protocol. In summary, sensitized patients can obtain a significant survival benefit by undergoing desensitization followed by kidney transplantation compared to alternative options, but risks of infection are higher and more studies are needed to help identify patients at greatest risk of dying and suffering from complications like malignancy or cardiovascular disease.
Tuesday, August 16, 2011
Urinalysis - concentration

Here are some simple facts about urine specific gravity, osmolality and their determination from the urinalysis.
Specific gravity
The reference substance for comparative purposes is water, which therefore has a specific gravity of 1.000. The so-called normal ranges are completely dependent on the amount of fluid ingested and solute excreted. Therefore, for example, uncontrolled diabetes mellitus may have a high urine specific gravity (due to the high amounts of glucosuria), as well volume depletion states and proteinuric conditions. Low urine specific gravity may be caused by excessive fluid intake, diabetes insipidus and diuretics, which all cause a relatively dilute urine to be formed.
Proximal tubular injury (e.g. acute tubular necrosis) may interfere with urinary concentrating capabilities, leading to isosthenuria (a specific gravity of 1.007-1.010). This is a set of circumstances whereby the final urine concentration is essentially equal to that of the glomerular filtrate produced at the early proximal tubule.
Note there are potential false elevations in urine specific gravity, many of which are caused by radiographic dyes, which can produce readings >1.03, if measured at a time close to the procedure.
Specific gravity measured by urine dipstick
The reagent strip in the usual urine dipstick actually measures the ionic concentration of urine. The free ions react with a pH indicator in the strip, thereby causing a change in colour, corresponding to the amount of solute present. Obviously, certain molecules may dissociate more freely than others, which can affect how easily they are to detect.
Urine osmolality
Osmolality differs from the specific gravity in that it depends on the amount of solutes, not on their molecular weight. The range of potential osmolality that can be produced by the normal kidney ranges from around 80-1200 mOsm/Kg. In normal circumstances, the approximate relationship between urine specific gravity and osmolality is 350mOsm/kg per 0.01 unit change in specific gravity (e.g. a specific gravity of 1.01 is equivalent to a urine osmolality of 350mOsm/kg). However, this relationship breaks down in the setting of larger molecular weight compounds, like glucose, certain antibiotics and radiocontrast material.
Monday, August 15, 2011
Methylene blue and refractory hypotension

Saturday, August 13, 2011
FSGS: The Basics
 Gearoid did a nice job reviewing the recent exciting findings surrounding primary Focal Segment Glomerulosclerosis (FSGS) and I thought it would be a good opportunity to review some of the sometimes confusing terminology and clinical basics.
Gearoid did a nice job reviewing the recent exciting findings surrounding primary Focal Segment Glomerulosclerosis (FSGS) and I thought it would be a good opportunity to review some of the sometimes confusing terminology and clinical basics. To start, it's helpful to realize that the term "FSGS" is used both by pathologists and clinicians to describe related but different things.
In the pathology world FSGS is a nonspecific renal biopsy finding (there are multiple pathophysiologies that can lead to it).
A useful way to understand the key light microscopy findings is to break the name down stepwise. Glomerulosclerosis refers to an increased glomerular extracellular matrix with obliteration of the glomerular capillary lumen. You can see this in the image at the top where on the right side of the glomerulus the capillary loops are open and over on the left they're mostly filled in with pink extracellular material.
The pattern of glomerulosclerotic distribution in FSGS is focal and segmental. The term focal (as opposed to diffuse) refers to only some glomeruli displaying changes while the term segmental (as opposed to global) refers to portions of an individual glomeruli being involved rather than the entire glomerulus which again, you can see nicely in the top image.
To make matters more complex there are five recognized FSGS histologic subtypes:
1. Not Otherwise Specified (NOS) variant
2. Collapsing variant
3. Tip variant
4. Cellular variant
5. Perihilar variant
Each of these has pathogenic and prognostic implications which we'll leave for another post.
In the clinician's world the FSGS histologic pattern is seen in the disease states of primary (or idiopathic) FSGS and secondary FSGS which has multiple associated etiologies (genetic, infectious, drug induced, nephron loss from a variety of other renal diseases and so on). Distinguishing between primary and secondary forms is important because while primary disease is treated with immunnosuppressives, secondary disease treatment involves general CKD management and addressing the associated illness.
Clinically primary FSGS often presents with the insidious onset nephrotic syndrome. Microscopic dysmorphic hematuria is found in roughly half of cases. At presentation, around a third have hypertension and a quarter have impaired renal function. Individuals with continued nephrotic syndrome despite therapy often progress to the need for renal replacement therapy. Secondary forms of FSGS are less likely to present with nephrotic syndrome though exceptions do exist (eg HIV associated secondary FSGS with collapsing variant histology).
The image at the top is taken from the July NephSap which, if you haven't seen it yet, is a fantastic must read. For ASN members it's available online here.
Thursday, August 11, 2011
Guyton was right all along: The primacy of the kidneys (and NaCl) in causing essential hypertension
 Dr. Arthur C. Guyton (1919-2003) was one of the greatest physiologists of our time. One of Dr. Guyton’s many seminal contributions to medicine was to establish the role of kidneys in long-term blood pressure regulation via a mechanism known as pressure natriuresis.
Dr. Arthur C. Guyton (1919-2003) was one of the greatest physiologists of our time. One of Dr. Guyton’s many seminal contributions to medicine was to establish the role of kidneys in long-term blood pressure regulation via a mechanism known as pressure natriuresis.
Mind the Gap

As Nate mentioned in a previous post, the urinary anion gap is helpful in differentiating whether a non-gap acidosis is of renal or extra-renal origin.
Urinary Anion Gap = Na + K – Cl
Because the major cation in the urine is NH4, this gives you a rough estimate of the NH4 level. In the setting of a distal RTA, the urine NH4 should be low and therefore there should be a positive anion gap. The problem with this test is that if there is some other unmeasured anion (e.g. ketoacids or hippurate following glue-sniffing) or even if the patient’s diet leads to significant changes in PO4 or SO4 excretion, it can be very inaccurate. One alternative suggested by Mitch Halperin is to measure the urinary osmolar gap. This is more useful because it detects the NH4 excretion regardless of the anion that is excreted along with it.
Urine Osmolar Gap = measured Uosm – calculated Uosm
Calculated Uosm = 2(Na + K) + Urea (mmol) + Glucose (mmol)
Because the other major cation in the urine is NH4 and this must be matched by an accompanying anion, most of the gap is therefore made up of NH4, giving you this formula.
Urinary NH4 = Urinary Osmolar Gap/2
The osmolar gap must be divided by two in order to account for the anion being excreted with NH4. Of course, this would all be easier if we could measure the NH4 directly. In our hospital, the assay for measuring NH4 is an enzymatic method using glutamate dehydrogenase. The lab was not able to give me a specific answer as to why they could not use this test on urine but looking around the net, it appears that it is not useful for measuring large quantities of NH4. The normal value in the serum is <35 µmol/L while in the setting of a metabolic acidosis, urine levels should be >200 mmol/day, orders of magnitude higher. So it seems for the moment that we are stuck with the osmolar gap as the best estimate in many hospitals.
Ref: Fluid, Electrolyte and Acid-Base Physiology, Halperin 2010
Monday, August 8, 2011
A suPAR breakthrough in FSGS

Wednesday, August 3, 2011
Cardiovascular mortality after transplantation - Are we doing a good enough job?
 The evaluation of a dialysis patient for possible kidney transplantation is a great challenge. On one side, we know that if the patient continues on dialysis, his yearly mortality will be around 20%. On the other hand, kidney transplantation carries a relative higher risk in the first year after transplantation and only those that survive over the first year will truly benefit from it. There is a classic paper from Wolfe et al. that shows when the line is crossed to the benefit side (Figure 1) in average.
The evaluation of a dialysis patient for possible kidney transplantation is a great challenge. On one side, we know that if the patient continues on dialysis, his yearly mortality will be around 20%. On the other hand, kidney transplantation carries a relative higher risk in the first year after transplantation and only those that survive over the first year will truly benefit from it. There is a classic paper from Wolfe et al. that shows when the line is crossed to the benefit side (Figure 1) in average.Nonetheless, more than 45% of kidney allografts are lost due to death with a functioning graft, a striking number that wonders if we are doing a good enough job in selecting our potential recipients. The main cause of death after transplantation is cardiovascular disease and transplant recipients are at increased risk due to both traditional as well as transplant-specific risk factors (Figure 2).
In fact, recent analysis suggested that the traditional CV risk factors add little predictive value regarding development of coronary heart disease after transplantation. The most important transplant-specific predictors of cardiovascular events included duration of pre-transplant dialysis, new onset diabetes after transplantation and history of delayed graft function and/or acute rejection. Immunosuppressive drugs play a major role in some of these factors. In addition, advanced age, sex, race and obesity were also included to compose the PORT risk score, which predicts the probability of developing coronary heart disease after transplantation. This score performed better than the Framingham equation in the transplant population with a c-Statistic value of around 0.8.
In addition to patient selection,
 the post-transplant medical care is essential. A surprising observation came from Dr Gaston and colleagues' work that evaluated the use of cardioprotective medications in kidney transplant recipients. Fewer than 30% of hypertensive patients with CV disease or diabetes were taking an ACEI/ARB 6 months post-transplantation, the use of aspirin was uncommon and statins were only prescribed in half of patients. We know that there are challenges with the use of ACEI/ARB in the initial period after transplantation due to its potential confusing effect with creatinine elevation. A meta-analysis comparing the effect of these agents with the traditional calcium channel blocker (amlodipine) showed that ACEI/ARB were associated with a decrease in GFR and a lower hematocrit, though proteinuria was significantly decreased and potassium levels were not affected.
the post-transplant medical care is essential. A surprising observation came from Dr Gaston and colleagues' work that evaluated the use of cardioprotective medications in kidney transplant recipients. Fewer than 30% of hypertensive patients with CV disease or diabetes were taking an ACEI/ARB 6 months post-transplantation, the use of aspirin was uncommon and statins were only prescribed in half of patients. We know that there are challenges with the use of ACEI/ARB in the initial period after transplantation due to its potential confusing effect with creatinine elevation. A meta-analysis comparing the effect of these agents with the traditional calcium channel blocker (amlodipine) showed that ACEI/ARB were associated with a decrease in GFR and a lower hematocrit, though proteinuria was significantly decreased and potassium levels were not affected.Despite their conclusion that there were insufficient data to determine the effect of ACEI/ARB on patient or graft survival, I strongly believe that extrapolating the literature from other populations is necessary due to the poor quality of trials so far. Diabetics and patients with proteinuria or history of heart failure/myocardial infarction would, in particular, benefit from those agents, in addition to statin/ASA once their kidney function has stabilized after transplantation. Highly opinion-based for now but hopefully new trials will be addressing this important question soon and meanwhile there is lots of room for improvement in the cardiovascular care after transplantation.
Monday, August 1, 2011
GnRH analogues for prevention of premature ovarian failure
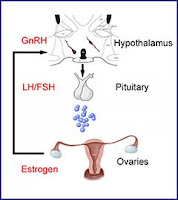
Various glomerulonephritides unfortunately often require treatment with chemotherapeutic agents, such as cyclophosphamide. A potential adverse effect of these agents in females of child-bearing age, is premature ovarian failure. Nate previously posted about this. Since then, there have been a couple of papers published that address this dilemma further.
A meta-analysis of six randomized controlled trials reported that GnRH treated women had a higher odds of return of spontaneous menstruation than those not treated (OR 3.46; 95% CI 1.13 – 10.57), but this did not translate into a significant difference in the rate of spontaneous pregnancy after chemotherapy. However, many people are cautious in the interpretation of these results, given the small sample size in the trials, heterogeneity of protocols and varying follow-up times.
More recently, a randomized trial of triptorelin vs standard care in women with breast cancer was published. This study, performed in Italy, randomized 133 women to GnRH and 148 to standard care. Subjects were reviewed one year after the last cycle of chemotherapy for occurrence of early menopause (defined as no resumption of menstrual bleeding and postmenopausal levels of FSH and estradiol one year after cessation of chemotherapy).
The rate of early menopause was 25.9% in standard care vs 8.9% in the GnRH group (p for difference <0.001).
Whilst encouraging, these findings must be tempered by the fact that recovery of menses does not directly translate to fertility (limited data was available for this cohort at the time of publication). Furthermore, the chemotherapeutic regimens used, dosing and the duration of treatment make it hard to generalize the findings to a ‘nephrology disease’ population. There are alternative methods available, with somewhat stronger recommendations from experts in the field – these include embryonic cryopreservation.


















